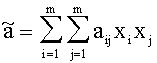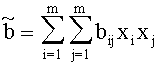Karlīs Nitrox Seite
Gas Logistic for Nitrox- Diver
Diving  etc. means filling the Bottles with a Gas different than Air.
etc. means filling the Bottles with a Gas different than Air.
This Website can not serve you with information about regulations for all four corners of the earth
, so it only speaks about technical aspects.
I strictly recommend to qualify as gas-blender before blending gases.
 Take care that tanks only get filled with gases they are rated, cleaned and labelled for.
Take care that tanks only get filled with gases they are rated, cleaned and labelled for.
For most Divers getting a fill with Nitrox or any other gas is just walking into the diveshop and ordering the fill they want. But it is always good to understand how something works.
Nitrox Fills
- Boosting
starts with decanting, but afterwards an oxygen-compatible pump is used to push the pressure to the requested value.
- Denitrogenizing
is filling with a special compressor. It works continuously and independent like normal air filles. The difference between a Compressor with Denitrogenizer and a normal Air Compressor is that the Compressor is Nitrox rated and that a Nitrox-Membrane works after the first stage of the compressor. The Membrane principle is like you know from osmosis: Nitrogen can pass it faster (or slower) and is so partly send back to the environment. A Denitrogenizer can be adjusted, so you can use it for all filles between "EAN21"  and (for example) EAN40.
and (for example) EAN40.
- Blending by Constant Flow
is filling with a Nitrox rated compressor. In its inlet a constant oxygen flow is injected and so mixed with the fresh air to a normobar Nitrox that than is "inhaled" by the compressor. The constant flow unit looks like the one that is used in first-aid.
- Blending by Partial-Pressure
normally means first decanting oxygen into the bottle until a precalculated pressure is reached and than toping with (oil free) air to the requested value.
Let's say we want to fill an empty tank to 200bar EAN60, that means a Nitrox with 60% Oxygen. So all we need to calculate how much Oxygen is needed in addition to the Air that contains the Nitrogen. Let's do it the easy way: filling 40% N2 by the use of Air means adding 40%*200bar/78%=102,6bar Air, the rest (97,4bar) is the needed Oxygen. Ok, we have done some simplifications, but that was just an example to explain how 'Blending by Partial-Pressure' works.
- Blending by Weight
normally means first decanting oxygen into the bottle until a precalculated weight is reached and than toping with (oil free) air to the requested final weight. The advantage of blending by weight is that you have no problems with the fact that you handle real gases. If you use Partial-Pressure-Blending, Gases have to cool down before you can do the next step and calculating means doing or consulting Van-der-Waals calculations. Blending by Weight so is easier and faster, if you have the scales for it.
Oxygen Fills
Decanting
Boosting 
Argon Fills
Decanting
Boosting is easy to achieve, any compressor can be converted into an Argon-Booster by an expert.
a description for the behaviour of real Gases in the 10-6 bar to 103 bar Range. In the most cases it has a good performance (as long as the Isotherms are above the critical temperatures in the pV- Diagram; which is normally fulfilled for Helium, Nitrogen and Oxygen at diving applications, not for CO2, most of it is liquid at the pressure in a CO2-cartridge).
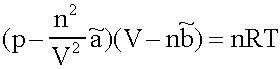
p:= Pressure, n:= Number of Mol's (a mol expanded gas means 22.4liter at 1bar), V:= Volume, 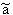 := Van der Waals-Coeffizient for attraction-effects,
:= Van der Waals-Coeffizient for attraction-effects, 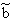 := Van der Waals-Coeffizient for repulsion effects (~volume of the atoms/molecules of the gas), R:= Gas-constant = 8.314 J K-1 mol-1, T:= Temperatur [Kelvin]
:= Van der Waals-Coeffizient for repulsion effects (~volume of the atoms/molecules of the gas), R:= Gas-constant = 8.314 J K-1 mol-1, T:= Temperatur [Kelvin]
For Gasmixes, that can be looked upon as a homogenous Gas (same Aggregate state, only Van der Waals-interrelations), the Van der Waals-Coeffizients can be estimated like this:
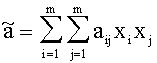
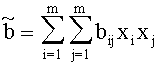
with xj, xi:= Molar-fraction of gas i and j, aij=(ai aj)1/2 and bij=(bi bj)1/2
|
Gas |
a [dm6 bar mol-2] |
b [10-2 dm3 mol-1] |
Mass [Gramm/22.4bar*l] |
|
O2 = Oxygen |
1.38 |
3.18 |
32 |
|
N2 = Nitrogen |
1.408 |
3.913 |
28.02 |
|
He = Helium |
0.034 |
2.37 |
4.0026 |
|
Ar = Argon |
1.363 |
3.22 |
39.95 |
|
CO2= Carbon-Dioxide |
3.640 |
4.267 |
44.01 |
Gas Analysis
has to be done (at minimum) twice when diving with mixed gases: 1) as part of the filling process, 2) prior to diving.
The predive analysis should be done by the diver, using instruments that are independent from those used during the filling.
When you are sure about the rest, a measurement of the oxygen-percentage is all what is needed.
Being sure about the rest is the other important thing. You surely do not want to get a chronically oil-poisoning, a carbon-monoxide poisoning, water or oil in your tanks, so always check that you get your fills at a responsible and competent Filling station.
 Nitrox-Index
Nitrox-Index
http://Rebreather.de/nitrox/gas_logistic.htm © Karl Kramer, 16.11.1998
 etc. means filling the Bottles with a Gas different than Air.
etc. means filling the Bottles with a Gas different than Air. etc. means filling the Bottles with a Gas different than Air.
etc. means filling the Bottles with a Gas different than Air.![]() Take care that tanks only get filled with gases they are rated, cleaned and labelled for.
Take care that tanks only get filled with gases they are rated, cleaned and labelled for.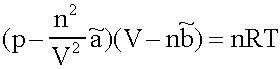
![]() := Van der Waals-Coeffizient for attraction-effects,
:= Van der Waals-Coeffizient for attraction-effects,![]() := Van der Waals-Coeffizient for repulsion effects
:= Van der Waals-Coeffizient for repulsion effects